
Today, it seems like everyone is talking about digitalization – and for good reason. Done properly, digitalization can unlock a whole host of benefits for companies including greater efficiency, cost savings, and the ability to do data analysis.
But in order to harness these rewards, digitalization needs to be carried out in a smart way – especially in the pharmaceutical industry where compliance and patient safety are the key drivers.
Digitalizing calibration
One area ripe for digitalization is calibration. Calibration is still largely paper based in the pharma industry, which means there is room for human error across the many steps required.
Process instrument calibration is just one of many maintenance-related activities in a manufacturing plant, and it doesn’t make sense for companies to use their limited resources and time performing unnecessary calibrations or following time-consuming, ineffective calibration procedures.
The use of paper for calibration also means that a huge potential resource – data from calibrations – is being wasted as it’s sitting in binders in a storage room rather than being easily available for analysis.
How automated calibration works
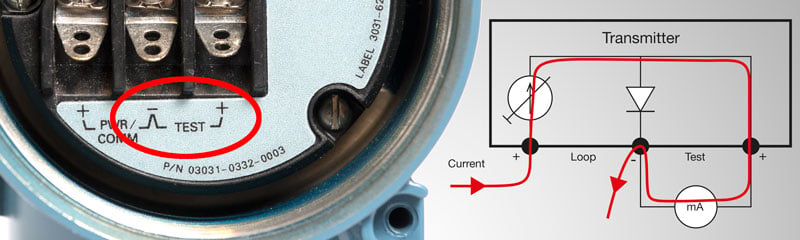
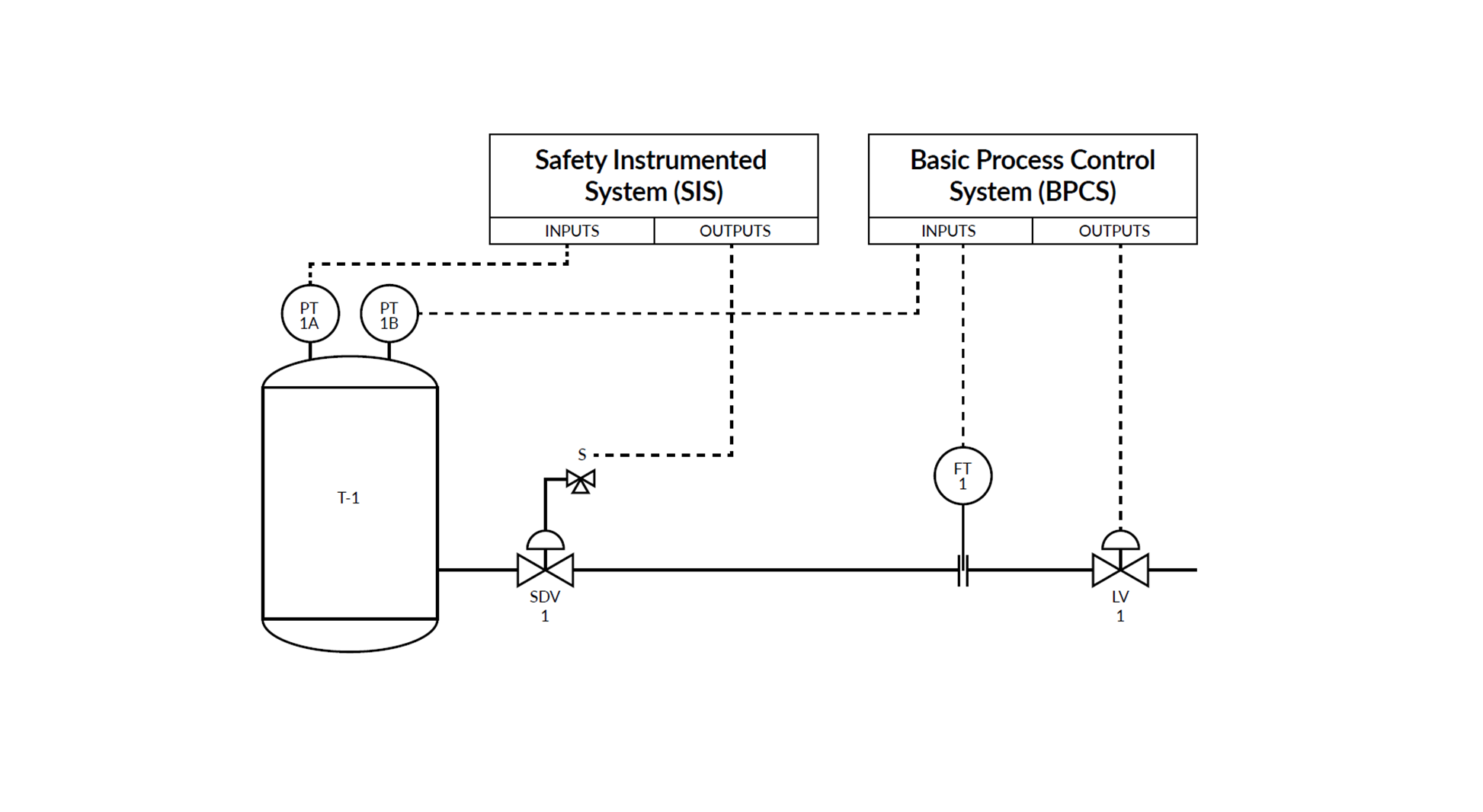
An integrated calibration solution is a smart way to digitalize calibrations.
Such a solution combines the actual calibrators, centralized calibration software, and industry knowledge to deliver an automated and paperless flow of calibration data.
This means moving away from resource-intensive manual data entry towards an automated system where everything is validated automatically by a single technician using a multifunctional device – in real time and with no room for human error.
The benefits
The benefits of digitalizing and automating calibration are numerous and include:
- Ensuring patient safety and compliance by ensuring that instruments are operating within tolerances
- Each calibration takes less time, improving operational efficiency
- Smart calibrators can provide guidance to technicians to decrease errors during calibrations
- Management can make more informed decisions based on current data
- The integrity of calibration data is kept safe in a tamper-proof central repository
- Data can be found quickly and easily for audit purposes
How to ensure successful digitalization
In order to make sure digitalization serves a useful purpose and fulfills its potential, several things are needed.
Firstly, the proper expertise to ensure that systems are in compliance with the Food and Drug Administration’s Good Manufacturing Practice (GMP) and other regulatory requirements. The GMP requirement 21 CFR Part 11, which regulates how the calibration certificate is documented and signed electronically, must be followed in order to create a compliant process.
Secondly, the actual calibration solution software and hardware need to be designed in a way that minimizes or removes the need for human input. This reduces the chance of error and removes the need for the “four eyes” principle – where a second set of eyes are needed to confirm calibration data is recorded correctly.
Finally, software tools need to be available to quickly access data, for example for audit purposes, as well as to carry out trend or other analysis on calibration data. This data can also be used to predict when a device is drifting out of tolerance to optimize maintenance, or for comparing performance between factories to optimize efficiency.
To find out more about what digitalizing calibration means in practice, read our white paper.
Related articles
- Tackling challenges in the pharmaceutical industry through digitalization
- Adapting to trends in the pharmaceutical industry
- How a trusted advisor can help pharma players maximize return on their digital investments
Related blog posts
- Future calibration trends by calibration experts in the pharmaceutical industry
- Automating the calibration management ecosystem
- CMMS and calibration management integration - Bridging the gap
- Calibration Trends Featuring Automation & Digitalization [Webinar]
- Data Integrity in Calibration Processes
- Calibration in Times of Digitalization - a new era of production
- The Evolution of Calibration Documentation
Download your copy of the Calibration Essentials Software eBook to learn more about calibration management and software.

















.jpg)

.png)
Discussion