
Safety and compliance are non-negotiable in the fine chemicals industry, which produces complex, pure chemical substances such as active pharmaceutical ingredients. Fine chemicals are batch driven, with complicated, multistage processes where accuracy and efficiency are critical.
In this industry, one of the keys to ensuring both safety and compliance is that measurements taken throughout the production process are accurate, which can be challenging to say the least with paper-based calibration. Paper-based calibration is time consuming and because it relies on manual data entry, prone to errors.
It’s commonly accepted that the typical error rate in manual data entry is about 1%, which while it might not sound like a lot can have huge implications for your calibration process. (Read more about in our blog post "Manual data entry errors".)
How can automated calibration help to address these challenges?
Read more about the benefits of automated calibration for the fine chemicals industry in our white paper (pdf format).
A paperless process to the rescue
Automated calibration solutions combine software, hardware, and calibration expertise to deliver an automated, paperless flow of calibration data with minimal need for manual data entry. This not only saves time and makes the process far more efficient, but it also helps to avoid mistakes typically associated with manual data entry – thus improving the quality and integrity of the calibration data.
Furthermore, calibration results are safely stored, tamper proof, and easily accessible in the calibration software for review, for example for audit or analysis purposes.
Safety, compliance, and continuous improvement
Removing human error reduces the chance that a production batch will be rejected due to out-of-tolerance calibrators and helps to ensure compliance with regulations like GMP and 21 CFR part 11.
Automating calibration processes also brings significant financial benefits. For example, if an instrument is found to be out of tolerance, at minimum it requires that the product is quarantined and subject to risk analysis and investigation. In the worst case, the entire batch will have to be discarded, increasing waste and leading to large financial losses.
What’s more, with calibration data in digital form rather than sitting in siloed paper archives it can be integrated with ERP systems, helping management to understand what’s going on and supporting better decision-making. And with everything in one easily accessible system, data across factories can be easily compared to spot trends and identify areas for improvement.
It’s important to remember that any automated calibration solution should be based on a thorough analysis of your specific needs to ensure the process is well designed and error-free. This is where working with a trusted advisor who can help analyze the process and find areas for improvement really pays off.
Read more about the benefits of automated calibration for the fine chemicals industry in our white paper.
Download the free pdf by clicking the picture below
Beamex calibration solutions
Learn more about Beamex products and services on our website or contact your local Beamex representative:
Related fine chemicals articles
- Safe and sound operations for fine chemicals
- Ensuring safety and sustainability in the fine chemicals industry
- How automated calibration changes the game in the fine chemicals industry
Related blog posts
- Automating the calibration management ecosystem
- Improving efficiency and ensuring compliance in the pharmaceutical industry
- CMMS and calibration management integration - Bridging the gap
- How a business analyst connected calibration and asset management [Case Story]
- Do more with less and generate ROI with an Integrated Calibration Solution
- Common Data Integrity Pitfalls in Calibration Processes
- Calibration management - transition from paper-based to digital
Download your copy of the Calibration Essentials Software eBook to learn more about calibration management and software.
















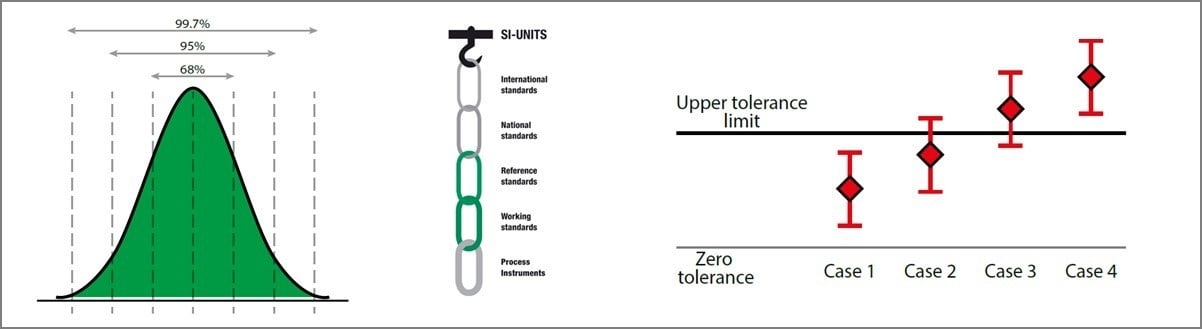
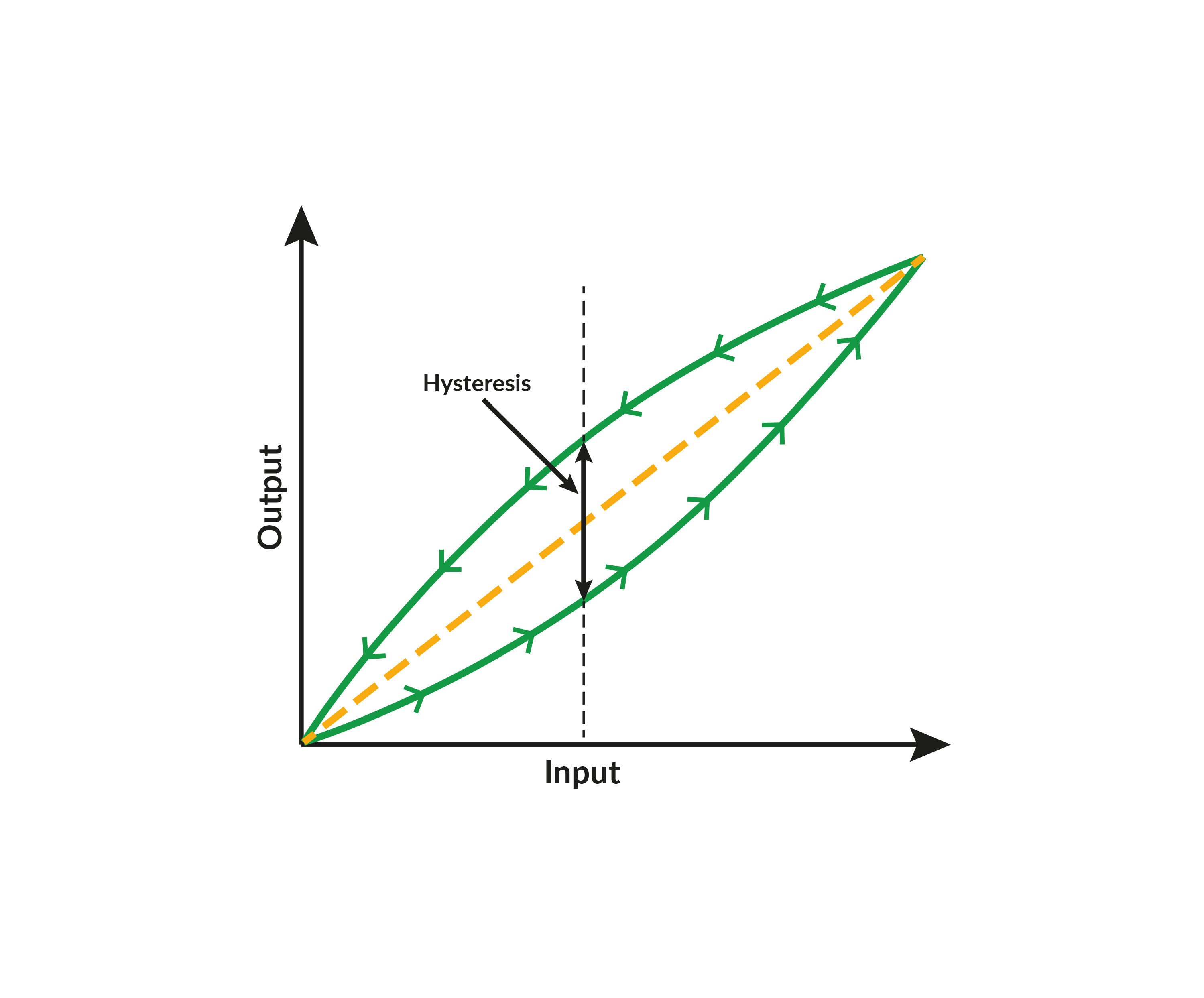






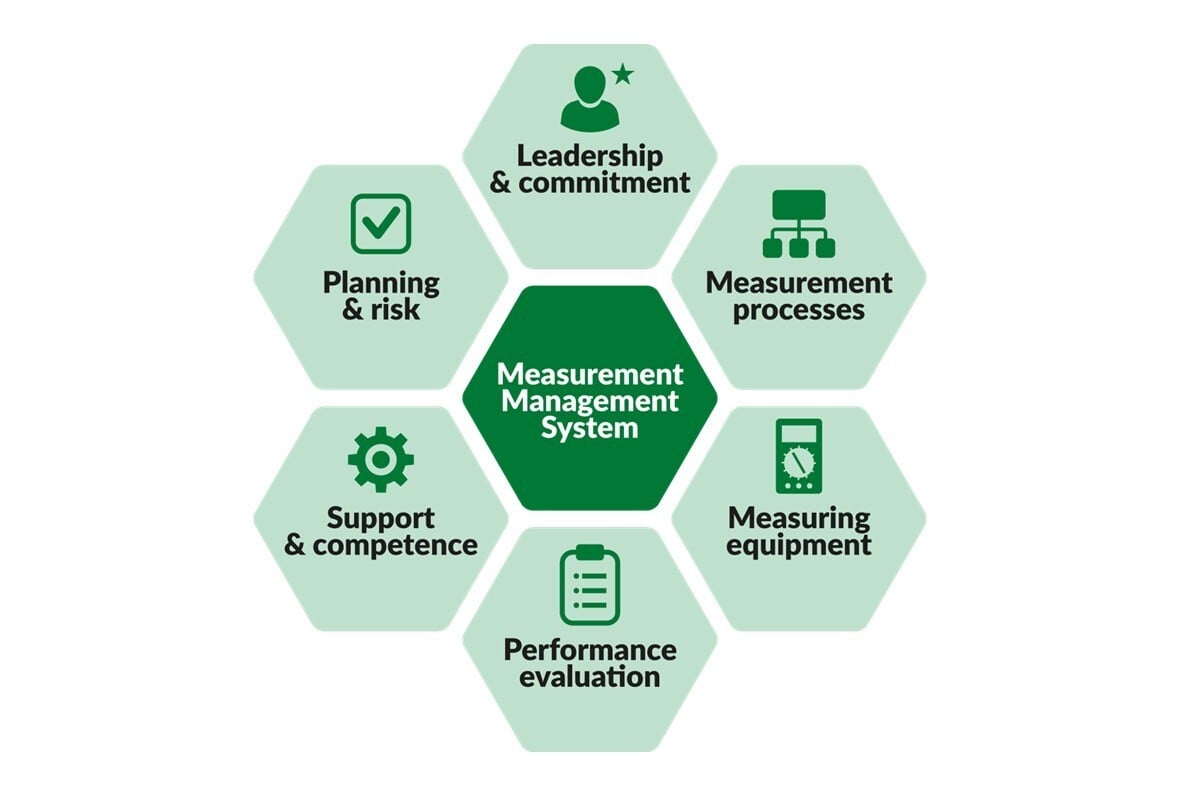

.jpg)
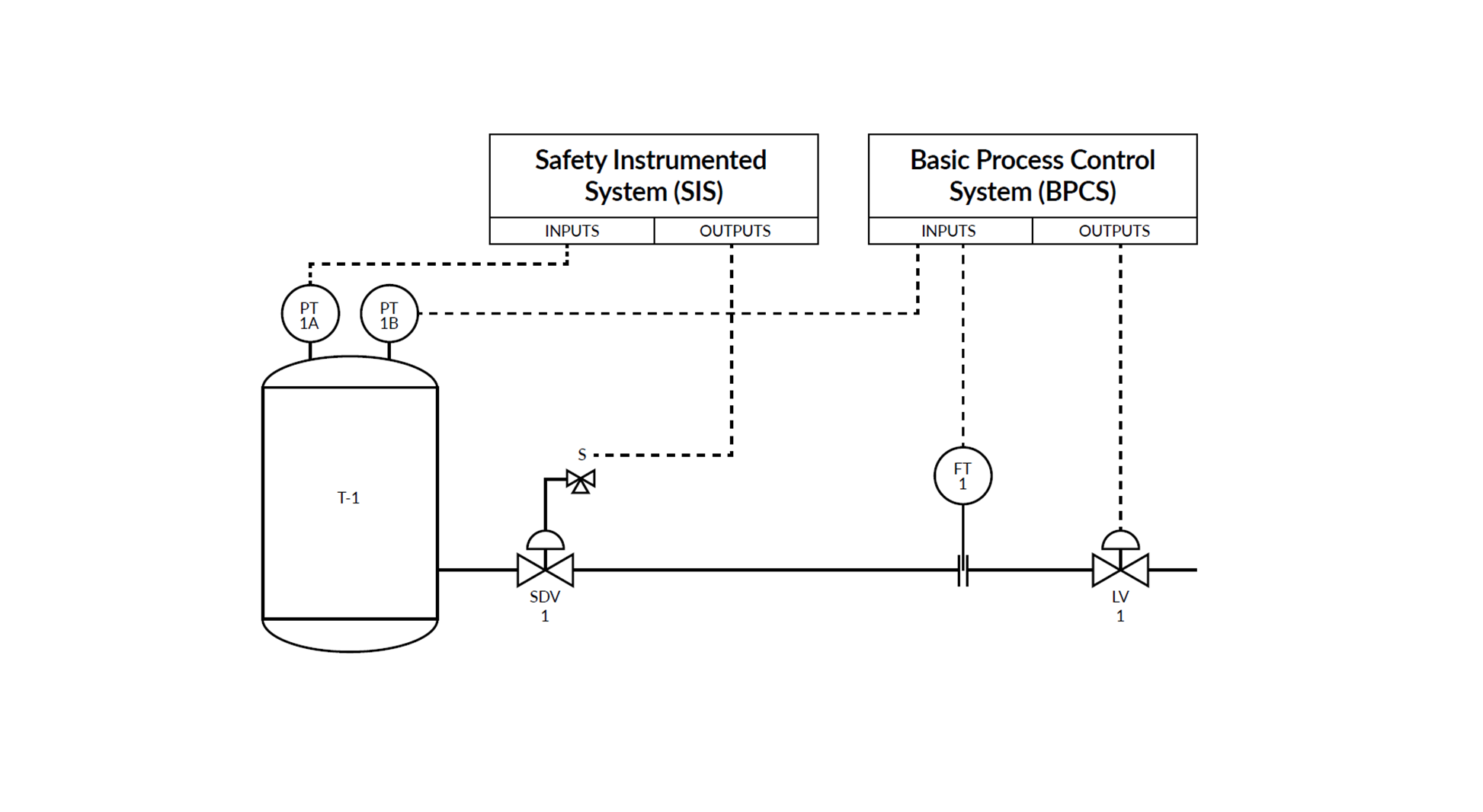


Discussion