
In this blog post, I will discuss the most common data integrity pitfalls in the calibration process.
Whether the calibration process is fully paper-based, or partially utilizing documenting calibrators, or even fully paperless, there are places in calibration processes where data integrity most commonly is jeopardized and needs special attention.
Data Integrity in brief
In one sentence: Data integrity is the maintenance of, and the assurance of the accuracy and consistency of the data over its entire life-cycle.
Although the Data Integrity is already a pretty old concept, it has become
ALCOA and ALCOA Plus are the key acronyms of Data Integrity. Learn more about these acronyms and other general information on data integrity in the earlier blog post titled Data Integrity in Calibration Processes.
What are the most common data integrity pitfalls?
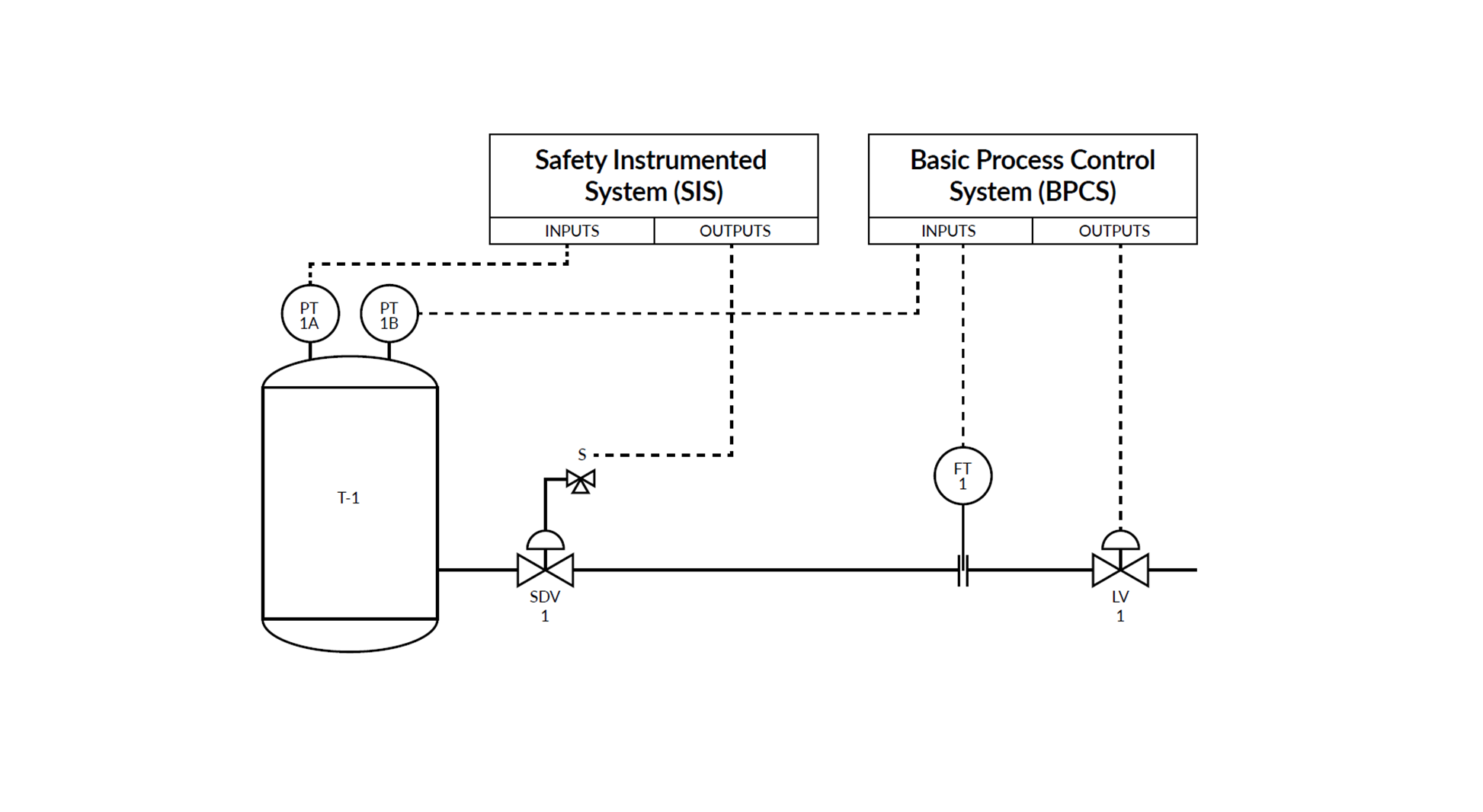
Let’s look at a normal calibration process in a pharmaceutical process plant and list some possible and most common places for data integrity issues. It does not matter if the calibration process is fully paper-based or paperless, similar issues need to be taken care of anyhow, either by a manual process or an automated system. Also, data integrity issues are similar in other industries, not only in pharmaceutical.
Some important aspects to be considered include, but not limited to, the following:
- User rights and passwords for any system is one obvious consideration. Every user needs to have the proper user rights and he needs to be authenticated by the system for access.
Some FDA warning letters indicate that there have been shared passwords, which is, of course, not acceptable. - An audit trail in an electronic system that keeps track of any modifications and records who did what with the help of an electronic signature that is required for any changes.
- In any kind of system, it should not be possible to delete, undo, redo or tamper with a single calibration point or the full calibration cycle, without a trace. It may be tempting to delete a calibration point or the whole result, if it was not good enough and try to redo it, but the original data of any calibration event needs to be recorded as it was originally witnessed. Of course, several calibration repeats can be done, if that is specified in the SOP, but none of the points/repeats representing the original data should be deleted. In a
paper based system, it may be difficult to fully control that, but also some electronic calibration systems allow the user to delete a bad calibration result and allow him to do it again until he achieves a satisfying result. - It must not be possible to edit, modify or tamper any original calibration data after it has been recorded, no matter what kind of system it is.
- It must not be possible to backdate any actions, neither accidentally or by falsifying the date and time. It may be tempting to backdate a calibration, in case you forgot to do it in time. In a paper-based system, it is difficult to control that you write the correct date, but also some electronic systems don’t have proper control of the date/time settings. Certainly, some users may have the rights to backdate a calibration date, for example in the case when a standard has been sent out for calibration and the calibration
date is updated when the unit is received. - A manual paper-based system may always have issues, such as a risk for typing errors, false interpretations of poor handwriting, loss of papers, etc. Often you look at a reading of a measuring device and write that reading on paper and then later type that result from paper into an electronic system. There are many places for typing errors in that kind of system and it is also time-consuming to do multiple manual data inputs. Modern electronic documenting calibration equipment will save the measurement results into its memory and transfer the result automatically into a computerized system for archiving, without any manual steps in between.
- All calibration records need to be archived and sometimes you need to analyze the older results and compare them with the new results. In case of a paper archive, this can be a really big job. With an electronic system, it is typically very easy to search/find the older records.
Outsourced calibration
Often some part of calibration work is outsourced to an external company. When calibration is outsourced you don’t typically have the same full control of the calibration process, as you have with internal calibrations, so it is a more uncontrolled process. In terms of data integrity, any part of the calibration work carried out by external resources should naturally follow the same principles as the internal calibration processes. If you send out a process instrument to an external calibration laboratory and get it back with a calibration certificate, you don’t have the same knowledge and control on how the calibration process was actually completed. Many of the calibration service companies don’t follow the same rules, regulations and good practices as, for example, pharmaceutical companies do. Certainly, if the pharmaceutical companies are outsourcing calibration work, they normally do audit the service companies. In some cases, external service companies can access the calibration system/software of a pharmaceutical company and work in the same way as internal workers.
Beamex calibration solution
For years, we have worked with multiple pharmaceutical customers and together we have recently enhanced our calibration solution to fulfill the updated regulatory requirements and the requirements and wishes of the customers. Already in the past, the Beamex calibration solution fulfilled the requirements of 21 CFR Part 11 and relevant regulations.
Now with our latest enhancements, the Beamex calibration solution further lowers the risk of ALCOA violations by identifying users on off-line mobile devices with their electronic signature and secures off-line data against potential data tampering. These mobile off-line devices include our MC6 and MC6-
Please visit Beamex web site or contact us for more information on our offering.
To download the related white paper "Common Data Integrity Pitfalls in Calibration Processes", please click the picture below:
















.jpg)


.png)
Discussion