Is it a leak? - Understanding the adiabatic process in pressure calibration
The adiabatic process is something we have all encountered if we have been working with pressure calibration. Often, we just don’t realize it, and we think there is a leak in the system.
In short, the adiabatic process is a physical phenomenon that causes the pressure media’s temperature to increase when we increase the pressure in a closed system. When we stop pumping, the media’s temperature cools down, and it will cause the pressure to drop – so it does indeed look like a leak in the system.
You can find many in-depth, complicated physical or mathematical explanations of the adiabatic process in the internet. But hey, we are not physicists or mathematicians, we are calibration professionals! Lucky you, you’ve got me to simplify this for you :-)
In this article I take a closer look at the adiabatic process, how to recognize and avoid it. A little bit of compulsory theory to start with and then diving into practical things.
If you are working with pressure calibration, you cannot miss this one!
Table of contents
- What is the adiabatic process?
- How do you know when it’s the adiabatic process and when it’s a leak?
- How to avoid the adiabatic process?
- Pressure media
- Conclusion
- Beamex's offering for pressure generation and calibration
What is the adiabatic process?
An adiabatic process is a thermodynamic change whereby no heat is exchanged between a system and its surroundings.
For an ideal gas undergoing an adiabatic process, the first law of thermodynamics applies. This is the law of the conservation of energy, which states that, although energy can change form, it can't be created or destroyed.
We remember from our school physics (well, some of us may remember!) the formula with pressure, volume and temperature, and how they depend on each other. Remember?
The combined gas law says that the relationship between pressure (P), volume (V) and absolute temperature (T) is constant. As a formula it looks as following:
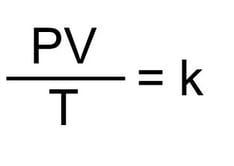
Where:
- P = pressure
- V = volume
- T = absolute temperature
- k = constant
OK, that did not yet take us too far, but please bear with me…
When using the above formula and comparing the same pressure system under two different conditions (different pressure), the law can be written as following formula:
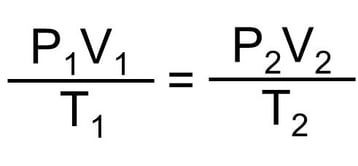 We can think of this formula as representing our normal pressure calibration system, having a closed, fixed volume. The two sides of the above formula represents two different stages in our system – one with a lower pressure and the second one with a higher pressure. For example, the left side (1) can be our system with no pressure, and the right side (2) the same system with high pressure applied.
We can think of this formula as representing our normal pressure calibration system, having a closed, fixed volume. The two sides of the above formula represents two different stages in our system – one with a lower pressure and the second one with a higher pressure. For example, the left side (1) can be our system with no pressure, and the right side (2) the same system with high pressure applied.
Looking at the formula, we can conclude that as the volume of a pressure calibration system remains the same, and if the pressure changes, then the temperature must also change. Or the other way around, if the temperature changes, then the pressure will also change.
The image below shows a typical pressure calibration system, where we have a pressure pump, pressure T-hose, pressure instrument to be calibrated (1) and pressure calibrator (2).
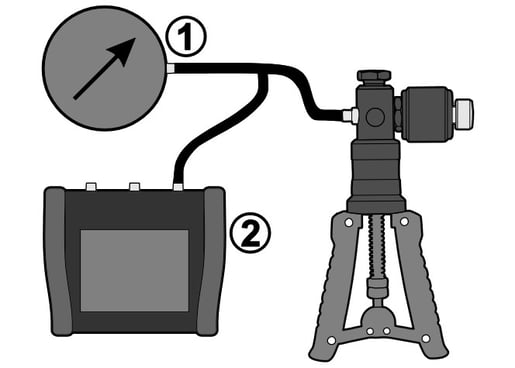
Typically, the volume of our pressure calibration system remains the same, and we change the pressure going through the calibration points. When we change the pressure (and the volume remains the same) the temperature of the medium will change. That’s physics, deal with it :-)
We can most commonly see the adiabatic process when we raise the pressure quickly with our calibration hand pump, causing the media (air) to get warmer. Once we stop pumping, the medium starts to cool down causing the pressure to drop - at first quickly, but then slowing down and finally stabilizing. This pressure drop looks like a leak in the system.
The same also happens with decreasing pressure – if we decrease the pressure quickly, the media gets colder. When we stop decreasing, the media will start to warm up, causing the pressure to rise. This may seem odd at first – how can the pressure rise by itself? Of course, the pressure does not increase a lot, but enough for you to see it and wonder what’s going on.
So, the adiabatic process works in both ways, with increasing and decreasing pressure.
The faster you change the pressure, the more the medium temperature will change, and the bigger effect you can see.
If you wait a while, the pressure media temperature will stabilize to the surrounding temperature and the effects of the adiabatic process will no longer be visible.
This is the essential learning from the adiabatic effect.
How do you know when it’s the adiabatic process and when it’s a leak?
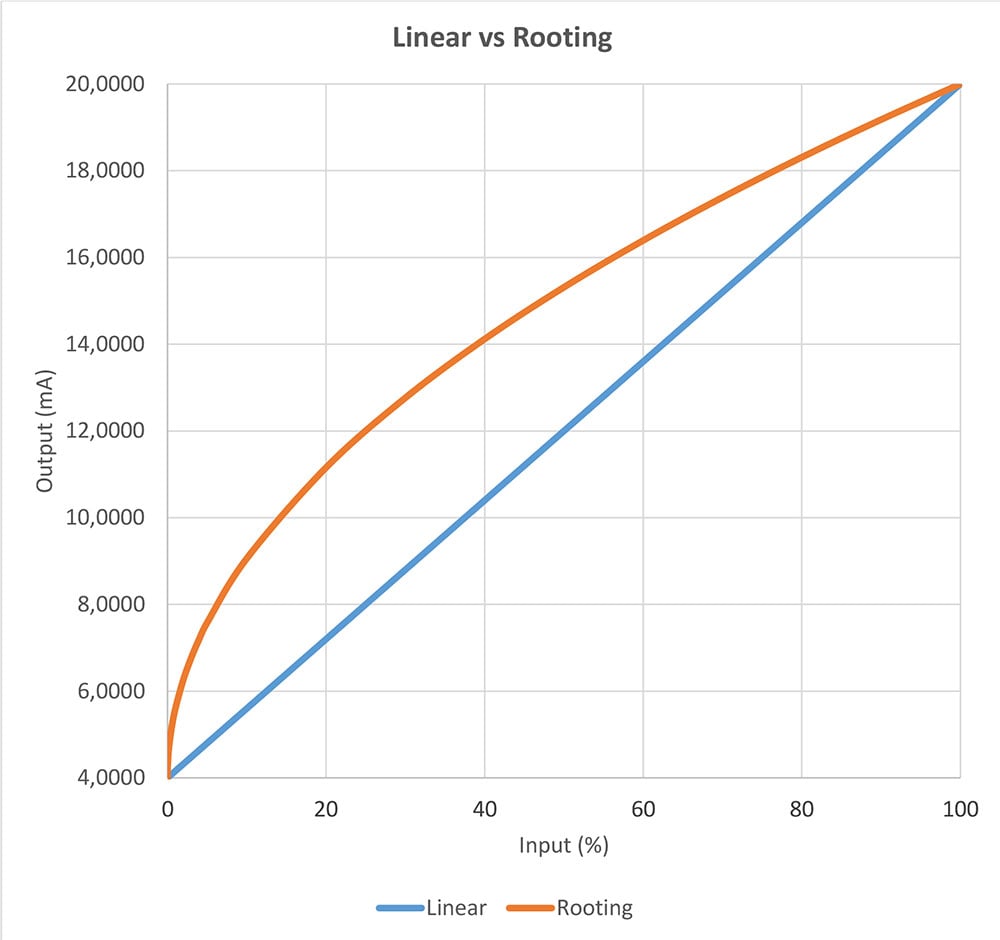
The main difference between the adiabatic process and a leak is that the pressure drop caused by the adiabatic process is bigger in the beginning, then slows down and disappears (stabilizes).
The pressure drop caused by a leak is linear and continues at the same rate.
The below image demonstrates the difference:
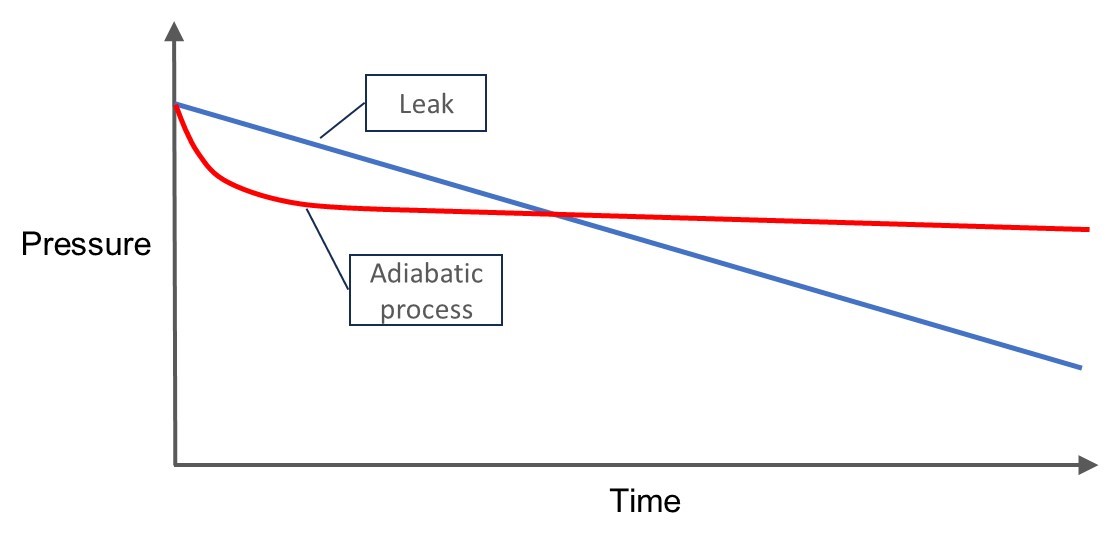 In the above image you can see how the pressure drop caused by the adiabatic process is first fast, but then slows down and eventually stabilizes (red line). While the pressure drop caused by a leak is linear (blue line).
In the above image you can see how the pressure drop caused by the adiabatic process is first fast, but then slows down and eventually stabilizes (red line). While the pressure drop caused by a leak is linear (blue line).
How to avoid the adiabatic process?
Pressurize slowly:
One of the easiest ways to minimize the adiabatic effects is to change the pressure slowly. By doing so, you allow the media more time to reach the same temperature as its surroundings, minimizing any temporary temperature changes. In practice, if you increase the pressure with a hand pump, and you step through several increasing calibration points, this may already be slow enough to avoid seeing the adiabatic process.
If you pump as quickly as you can up to 300 psi (20 bar), then you will most certainly see the effect of the adiabatic process.
Wait:
After adjusting the pressure, give it some time to stabilize. A minute or two should do the trick. This allows any temperature changes in the medium to reach equilibrium with the ambient conditions, and the pressure will stabilize accordingly.
Pressure media
You can also affect the adiabatic process with your choice of pressure media. In practice it is of course not always possible to change the media. Your normal hand pump uses the air as media. For higher pressure, you may use a hydraulic pump with water or oil as the medium.
The effects of the adiabatic process are generally more prominent in air or gas-operated calibration pumps than in hydraulic (water or oil) ones.
This is mainly due to gas being much more compressible, so the pressure increase will push gas molecules closer together, and this work done in gas is transformed into energy, causing heat. In addition , gas/air has lower thermal conductivity than liquids, so less heat is conducted away from gas.
Conclusion
In our service department, we regularly get questions about pressure pumps having leaks, while in most cases it turns out to be the adiabatic process that has made the customer think that there is a leak.
Understanding the adiabatic process and its impact on calibration pressure pumps is crucial for users to avoid misdiagnosing issues. By changing pressure at a moderate pace and allowing adequate time for stabilization, you can achieve more accurate and consistent results.
Beamex's offering for pressure generation and calibration
At Beamex, we have a lot to offer for pressure calibration.
For example, our PG range of calibration pumps for pressure generation, the ePG electric pressure pump, the POC8 automatic pressure controller, and a number of pressure calibrators.
Don't forget our calibration software offerings, and the entire calibration ecosystem.
We also offer expert services and training services for pressure calibration.
You can also download a free pressure calibration eBook, and visit the handy pressure unit converter on our website.
Please feel free to contact us to discuss on your pressure calibration challenges and how we can be your partner for calibration excellence.
Please scroll through the carousel below for more interesting articles related to pressure calibration!















.jpg)


Discussion